On June 5, 1981, the U.S. CDC published a Morbidity and
Mortality Weekly Report. In this report detailed five different cases of a rare
lung infection, Pneumocystis carinii,
in previously healthy young males, indicating severe immune deficiency. By the
end of the year, there are over 270 reported cases of similar immune deficiency
among young men. This immune deficiency would quickly be identified as the
ultimately fatal Human Immunodeficiency
virus.
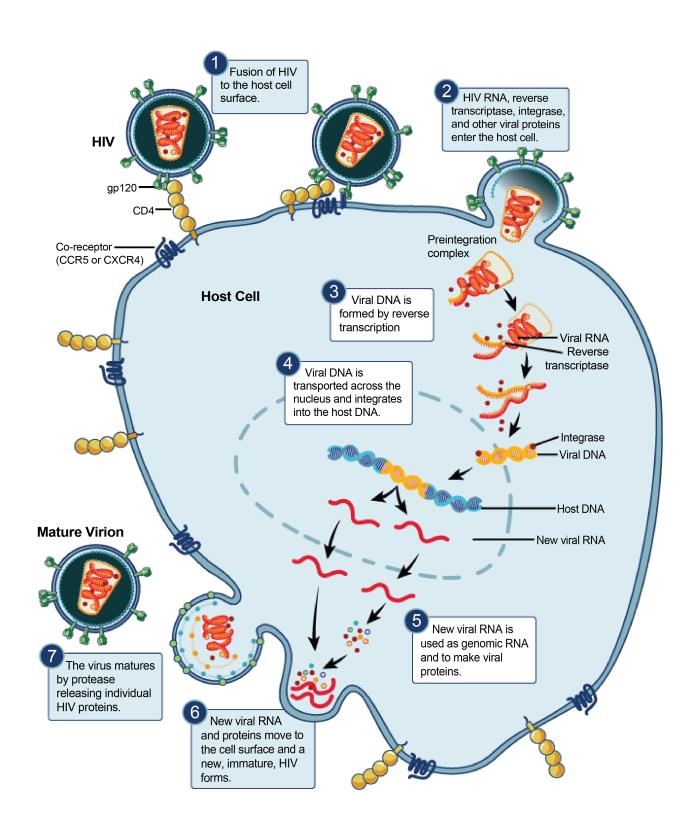
 The Human Immunodeficiency virus (HIV) is a retrovirus that
causes Acquired Immunodeficiency Syndrome (AIDS). This is a condition in humans
that leads to ever-increasing immune system failure. Without treatment, the
average survival time after infection is around 10 years. The human
immunodeficiency virus is transferred via body fluids, and can only infect
humans. It infects the immune system by targeting CD4+ helper T cells. As the
infection progresses, apoptosis of CD4+ T cells decline their numbers to a
critical level, leaving the body increasingly susceptible to opportunistic
infections.
The Human Immunodeficiency virus (HIV) is a retrovirus that
causes Acquired Immunodeficiency Syndrome (AIDS). This is a condition in humans
that leads to ever-increasing immune system failure. Without treatment, the
average survival time after infection is around 10 years. The human
immunodeficiency virus is transferred via body fluids, and can only infect
humans. It infects the immune system by targeting CD4+ helper T cells. As the
infection progresses, apoptosis of CD4+ T cells decline their numbers to a
critical level, leaving the body increasingly susceptible to opportunistic
infections. |
| https://www.aids.gov/hiv-aids-basics/hiv-aids-101/statistics/ |
Today, the CDC reports that more than 1.2 million people in
the United States currently live with an HIV infection, with nearly 1 in 7
unaware of their infection. There are currently 31 antiretroviral drugs (ARVs)
with FDA approval to treat the HIV infection. However, none of these treatments
cure or prevent HIV or Aids.
A recent study published in Nature examines the challenges
of developing a HIV vaccine. One of the largest obstacles is the identification
of mechanisms of protective immunity. Roederer et al., 2014 created a
comparable model with simian immunodeficiency virus (SIV) in Indian origin
rhesus macaques.
SIV is nonpathogenic in their natural hosts, however when an
Asian or Indian rhesus macaque is exposed, the animal will develop simian AIDS
(SAIDS). Virus strains from primate species are believed to have crossed the
species barrier into humans, resulting in HIV-1 and HIV-2. The similarities
between HIV and SIV make trials with the rhesus macaque ideal for vaccination
studies in an organism comparable to humans.
Previous studies have given promising indications that among
the five current human efficacy trials of HIV-1 vaccines, one has shown
moderate success. The RV144 trial has been the most promising, however its
efficacy has only been reported to be around 31%. Another trial, HVTN505, was
predicted to be very effective in protecting against HIV, was halted for
futility with no observed vaccine efficacy.
 |
| http://phil.cdc.gov/phil/details.as |
In an attempt to study why these different human trials have
not been successful, Roederer et al. examined SIV. This study utilizes a
nonhuman primate model with a high amount of acquisition endpoints that are
similar to human efficacy studies of HIV. This primate model was able to test
and confirm that an envelope-elicited immune response to the virus is necessary
and sufficient to protect against acquisition of the infection. In this study,
SIV environment T cell mosaic immunogen (comparable to human helper T cells ) elicited more effective general responses, but
less effective antibody responses. This study also was able to sequence and identify a sequence
signature in the SIV envelope, which is possibly shared by HIV in humans, which
can program the neutralization phenotype of viruses, affecting the entire
surface of the protein.
This study examined three different vaccine groups of Indian rhesus monkey, and
concluded that there was no association between protection from infection and
protection from pathogenesis. This suggests that when developing in humans,
responses that block initial infection are not the same cellular responses that
will control the pathogenesis of an already present HIV infection.
The analysis of the effectiveness of SIV, and its
comparability to HIV, enabled the study to provide insight into possible
failures of the human HVTN505 and why RV1444 has had only moderate success. Similar
vaccinations developed specifically for SIV envelope-expression vectors
protected against a subset of neutralization sensitive viral variants, but did
not generate an effective antibody response against specific variants. They are
able to conclude that HVTN505 failed due to its inability to elicit antisera
that completely neutralized circulating HIV-1 strains that are primarily
neutralization resistant.
In contrast, RV144 has had moderate success, indicating that
antibodies were successfully elicited and could neutralize some viruses
circulating in the cohort, but not the more resistant varieties of the
virus.
 |
| http://upload.wikimedia.org/wikipedia/commons/7/7f/Rhesus_Macaques_-_cropped.jpg |
Immune correlate studies that interrogate both virus
sequences and immune responses can provide key insights on mechanisms of
protection from HIV-1 acquisition. This study presented valuable insight into
the similarities in the SIV and HIV strains, as well as reasoning behind the
failure of various human efficacy trials of HIV vaccines.
Ref:
1) Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, et al. Immunological and Virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014; 505:502-508.
2) Center for Disease Control and Prevention http://www.cdc.gov/hiv/default.html/
2) Sharp P, and Hahn B. Origins of HIV and the AIDS Pandemic. Cold Spring Harbor Perspectives in Medicine 2011; 1.1.
4) AIDS. gov https://www.aids.gov/
This article goes really well with the other HIV vaccine article. It makes me wonder if the small sub-unit process talked about in the other article could possibly be useful in this situation. It would also be interesting to see if the SIV aspect of treatment could be combined with the sub-unit component of the other article to make a better vaccine.
ReplyDelete