Distinguishing
between the reality and imaginary is something most healthy humans can do
without much of an issue. However, schizophrenia is a harmful mental disorder
where an individual often has trouble with this distinction. As an example, one
schizophrenic was asked to draw a self portrait, and this is what he drew:
 |
|
|
Unless this
individual was a purple man hooked up to a machine with math equations written
on his body, it’s apparent that this individual has a warped perception of
reality. Schizophrenia is a harmful, chronic, socially detrimental disease.
Often, individuals experience hallucinations, can hear imaginary voices inside
their head, or believe in illogical delusions. Schizophrenics often lose touch
with reality; examples of symptoms include believing they are someone else,
attempting to smell or touch something that isn’t there, or difficulty in
communicating what they are thinking. While only 1% of Americans have this
illness, the risk of having it jumps much higher if there is a family history
of schizophrenia. However, more de novo mutations are being discovered where a
genetic change occurs without any family history of schizophrenia.
Understanding the origins of the disease can lead to future developments in
medicine and treatment to help those with the disease.
 |
|
Pink Floyd rock
n’ roll drummer Syd Barrett was famously thought to have the illness
|
No one gene
mutation by itself can cause schizophrenia. People with the disease generally
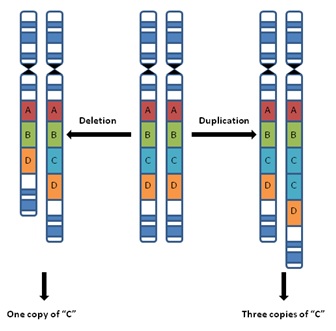
have copy-number variations (CNVs), where the DNA has an abnormal number of
copies. This means thousands of base pairs of DNA can be deleted, duplicated,
or somehow altered. Clearly, this leads to detrimental results where a human
can have drastically altered neural functions. In fact, schizophrenics likely
have other mental illnesses, issues with brain development, or malfunctions in
production of chemicals in the brain. De novo mutations especially link
schizophrenia with autism and other intellectual disabilities.
 |
|
Notable CNVs,
including deletion and duplication of alleles in a strand of DNA
|
The authors
Fromer et. al of the paper De Novo
Mutations in Schizophrenia Implicate Synaptic Networks, used a large DNA
sequencing study of de novo mutations in schizophrenics to determine if these
mutations caused changes in biological processes, what genes they changed, and
how it affected development of other neurological illnesses. They found several
synaptic protein-protein interactions in both the pre- and post-synaptic
(although the study focused on the post-synaptic proteins) affected by numerous
de novo schizophrenic mutations.
 |
|
|
Image a on the left shows the localization of
post-synaptic proteins affected by several de novo mutations. Notably, the NMDA,
AMPA and kainite receptors receptors are affected (shown with a upside down
purple triangle or an orange circle), which are necessary for healthy nervous
system function and are involved in breathing, learning, and memory. They bind
to glutamine and allow positively charged ions such as calcium to pass through
the cell membrane. These receptors are excitatory, meaning when glutamines bind
they will activate subsequent signaling cascades by producing second messengers.
In this example, the protein DLG2 is a member of this receptor family, and the
de novo mutation associated it is a loss of function mutation. Many signals
associated with proper learning and memory will thus be disrupted in an
individual with this de novo mutation.
Alternatively,
another notable de novo mutation affects the actin dynamic and stability
signaling cascade. These proteins play an important role in muscle contraction,
cell motility and cell shape and polarity. In schizophrenics, actin filament
mutations affect proteins that can change the shape and polarity of synaptic
receptors and can pass on the signaling cascade of the aforementioned NMDA
receptors. These mutations further affect cognitive ability and neurological
stability, leading to several mental illnesses and intellectual disabilities,
most notably autism.
Image b shows how the overlap in genes and
proteins due to de novo mutations can lead to further mental illnesses that
coexist with schizophrenia. The study focused on how these CNVs changing large
sections of DNA can also lead to autism, ADHD, and other intellectual
disabilities. Autism is a spectrum disorder involved with difficulty in social
interaction while individuals with ADHD generally have difficulties focusing
and paying attention. These are common symptoms seen in schizophrenics, so it’s
possible these illnesses are linked somehow. To see how, we should examine them
on the molecular level and the de novo mutations common in each disease.
Studies have
shown that these NMDA receptors also exhibit loss of function mutations in
autism and ADHD. In addition, genes such as SCN2A and POGZ also exhibited de
novo loss of function mutations in studies concerning all three diseases. SCN2A
is a gene involved in proper action potential propagation in voltage gated
sodium channels, while POGZ is necessary for mitosis and regulating neuron
proliferation. The overlaps in de novo mutations suggest that schizophrenia,
ADHD, and autism not only often occur simultaneously, but also share similar
disease mechanisms.
What does this
mean for the future of the disease? While future treatments cannot be formed
from these studies alone, they do provide a basis for thinking about mental
illnesses as a complex system in the body that cannot be thought of in a
vacuum. The similarities between the three illnesses in genetic mutations
suggest basing treatments around how these diseases interconnect with one
another, not each one as a separate entity.
References:
1.) Fromer M., Pocklington A.J., Kavanagh D.H., Williams J.H., Dwyer S., Gromley P., Georgieva L., Rees E., Palta P., Ruderfer D.M., Carrera N., Humphreys I., Johnson J.S., Roussos P., Barker D.D., Banks E., Milanova V., Grant S.G., Hannon E., Rose S. S., Cahmbert K., Mahajan M., Scholnick E.M., Moran J.L., Kirov G., De novo mutations in schizophrenia implicate synaptic networks. 2014. Nature. 506: 179-184
http://www.nature.com/nature/journal/v506/n7487/full/nature12929.html
2.) "Schizophrenia." NIMH RSS. National Institute of Health, n.d. Web. 12 Mar. 2015.
http://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml
3.) Blanke, Marie L., VanDongen A. M. J. "Activation Mechanisms of the NMDA Receptor." NCBI: Biology of the NMDA Receptor. U.S. National Library of Medicine, 2009. Web. 12 Mar. 2015.
http://www.ncbi.nlm.nih.gov/books/NBK5274/
4.) Dominguez R., Holmes K.C., Actin Structure and Function. 2011. Annual Reviews. 40: 169-186
http://www.ncbi.nlm.nih.gov/pubmed/21314430
5.) "Autism Fact Sheet." : National Institute of Neurological Disorders and Stroke (NINDS). N.p., Sept. 2009. Web. 11 Feb. 2015.
http://www.ninds.nih.gov/disorders/autism/detail_autism.htm
I thoroughly enjoyed this post and I was greatly intrigued by the idea of De novo mutations causing schizophrenia. It seems strange and somewhat frightening that changes in one area of the brain to a very specific protein can cause such drastic mental changes.
ReplyDelete